Our Business
Main Business Sectors of the Company
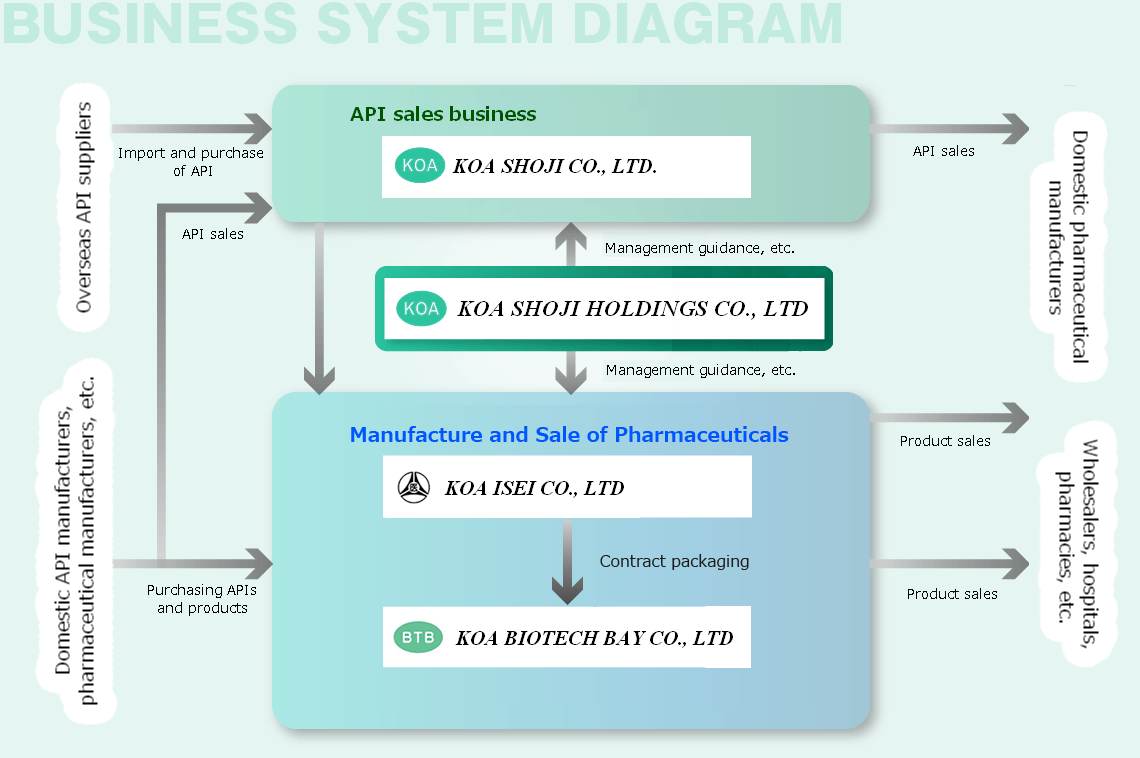
API Sales Business
APIs are the active ingredients of pharmaceuticals. KOA SHOJI is mainly engaged in the import and sale of APIs.
Manufacture and Sale of Pharmaceuticals
KOA ISEI and KOA BIOTECH BAY are engaged in the manufacture and sale of ethical pharmaceuticals and over-the-counter drugs, as well as the purchase and sale of pharmaceuticals and contract manufacturing services.

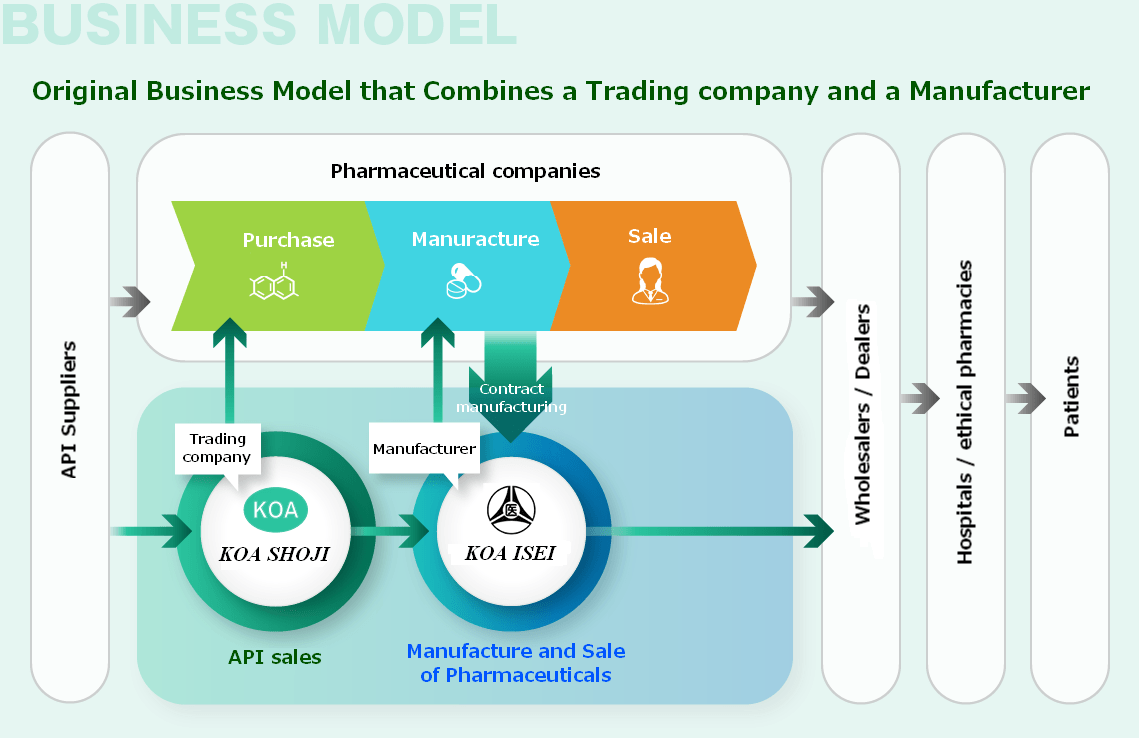
― Original Business Model that Combines a Trading company and a Manufacture ―
To be the best partner for generic manufacturers,
not only through import and sale of APIs,
but also product sale and contract manufacturing.
― New Business Model Innovation (Corporate Slogan) ―
Enhance group synergy
by combining a trading company and a manufacturer
― Group's Business Model ―
The original business model
that combines a trading company and a manufacturer
Characteristics of our Group's Business
1Positioning of the Group in the Pharmaceutical Industry
Our group is engaged in the import and sale of APIs, focusing on providing customers with high value-added, high-quality, and low cost imported APIs. We also manufacture and sell pharmaceutical preparations to meet our customers' needs. Additionally, we conduct business with a wide range of domestic and overseas pharmaceutical manufacturers based on a system that enables integrated operations from the procurement of APIs to the manufacture of pharmaceutical preparations. We are actively engaged in the manufacture and sale of self-developed products, as well as contract manufacturing for major Japanese manufacturers, and developing our business to meet the diverse needs of the pharmaceutical industry with a focus on generic drugs.
2Research and Development System
Regarding R&D activities, we proceed with development based on a list of generic drug candidates selected for each fiscal year. The basic policy of our R&D activities is the in-house development of generic drugs based on three categories: cancer, rheumatoid arthritis, and dialysis patients.
Drugs related to these three categories are mainly anticancer agents, antiemetic agents, pain relievers, agents for pruritic skin diseases, neuropsychiatric agents, agents for metabolic diseases, and anti-rheumatic agents.
We will consider outsourcing development to overseas pharmaceutical companies for items whose patents expire earlier in overseas markets than in Japan. This is done for patent advantages, such as progress in the development of new items, including technologies to avoid patents, or if we can shorten the development period for items such as oral drugs that are already distributed in overseas markets.
3Business Structure
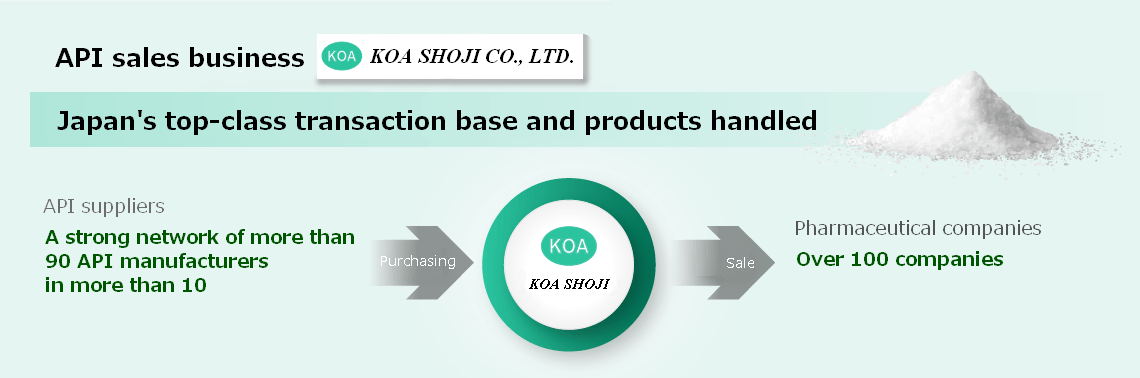
API Sales Business
Since its establishment, our group has been providing a stable supply of high-quality and low-cost imported APIs with high added value instilling confidence among customers to become the "Best Partner for Generics."
The manufacturing of APIs, which are raw materials for pharmaceutical products, is closely related to the patents of the relevant pharmaceutical products. Therefore, during the patent period, the APIs for the relevant pharmaceutical product is generally produced by the new drug manufacturer.
Regarding generic drugs, we believe that it is common for generic drug manufacturers to purchase APIs from other companies instead of manufacturing by themselves because patents have expired, and generic drug manufacturers, such as manufacturers and distributors, need to stock a wide range of generic drugs efficiently. Therefore, we supply API to generic manufacturers.
We have a strong network of more than 90 API manufacturers in more than 10 countries worldwide. Despite being a trading company, we have our own "Pharmaceutical Analysis Center" with the most advanced analytical equipment.
In addition to providing a stable supply of existing products, we also make proposals for new products and provide support from the R&D stage.
Specifically, we research the marketability, draft specifications and concepts, and select new sales items.
Once the item has been approved for use, each department, including development, sales, and quality assurance, must work together to obtain approval. Therefore, we manage the development schedule and item information in a unified manner and share information.
We also examine new synthesis and purification methods for APIs, propose quality improvements to API manufacturers, provide technology for new manufacturing methods, and provide technical support services to ensure stable supply of high-quality, low-cost APIs.
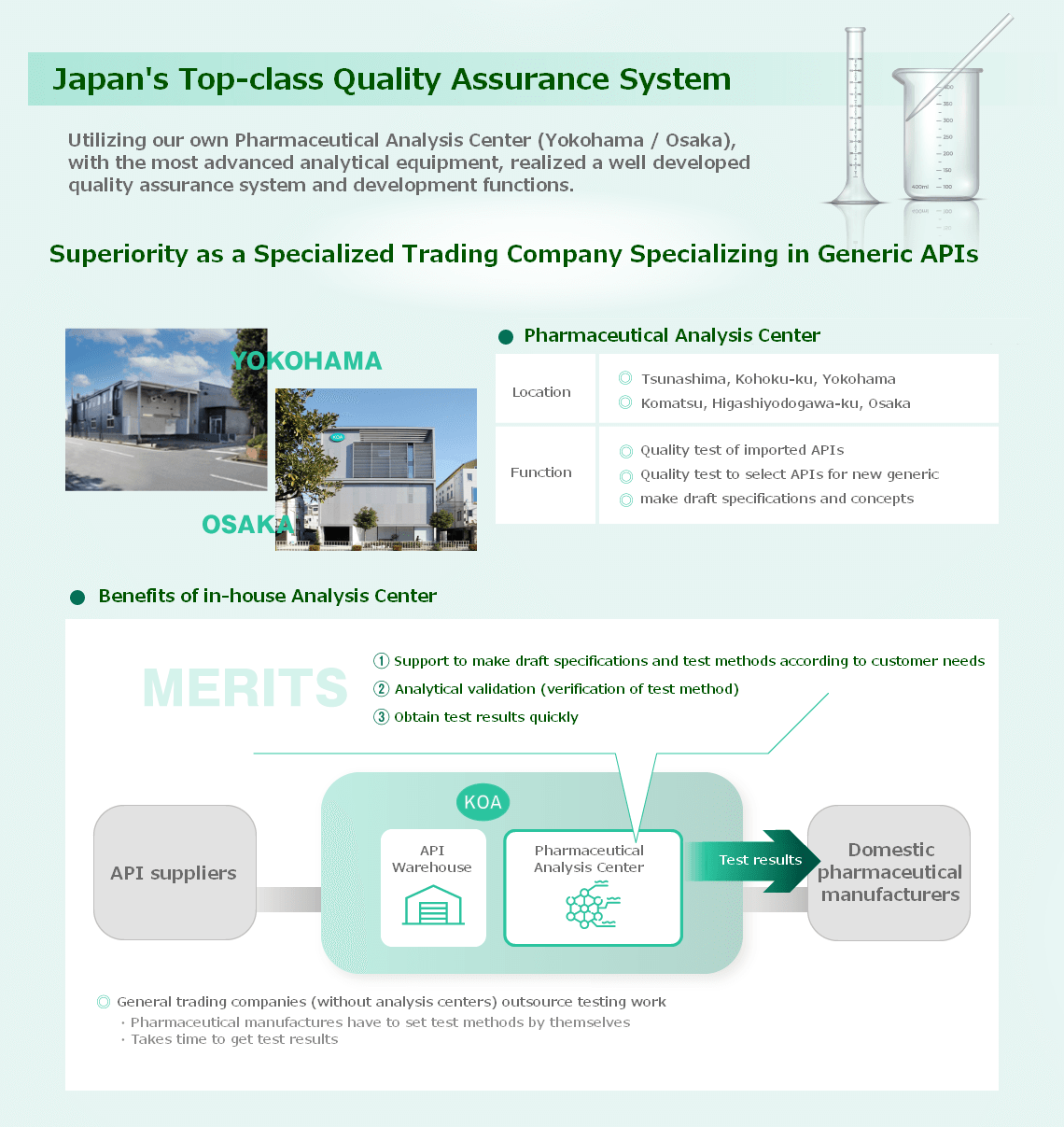
※For more information about our testing facilities, please refer to the Virtual Tour page.
Manufacture and Sale of Pharmaceuticals
In addition to the manufacture and sale of our own products, we are actively engaged in contract manufacturing for major Japanese manufacturers. To respond to the development of new generic drugs, we focus on contract development manufacturing organizations (CDMOs): contract manufacturing and development of pharmaceutical products from the development stage.
We are working to earn the trust of our customers by meeting the standards required by the good manufacturing practice (GMP) in Japan and improving our quality control capabilities, the most important factors in pharmaceutical manufacturing. Simultaneously, we can provide contract manufacturing services by possessing facilities that enable us to manufacture highly pharmacologically active injectable drugs that require advanced technologies in small quantities and in a wide variety of products that are difficult to handle by major manufacturers specializing in mass production.

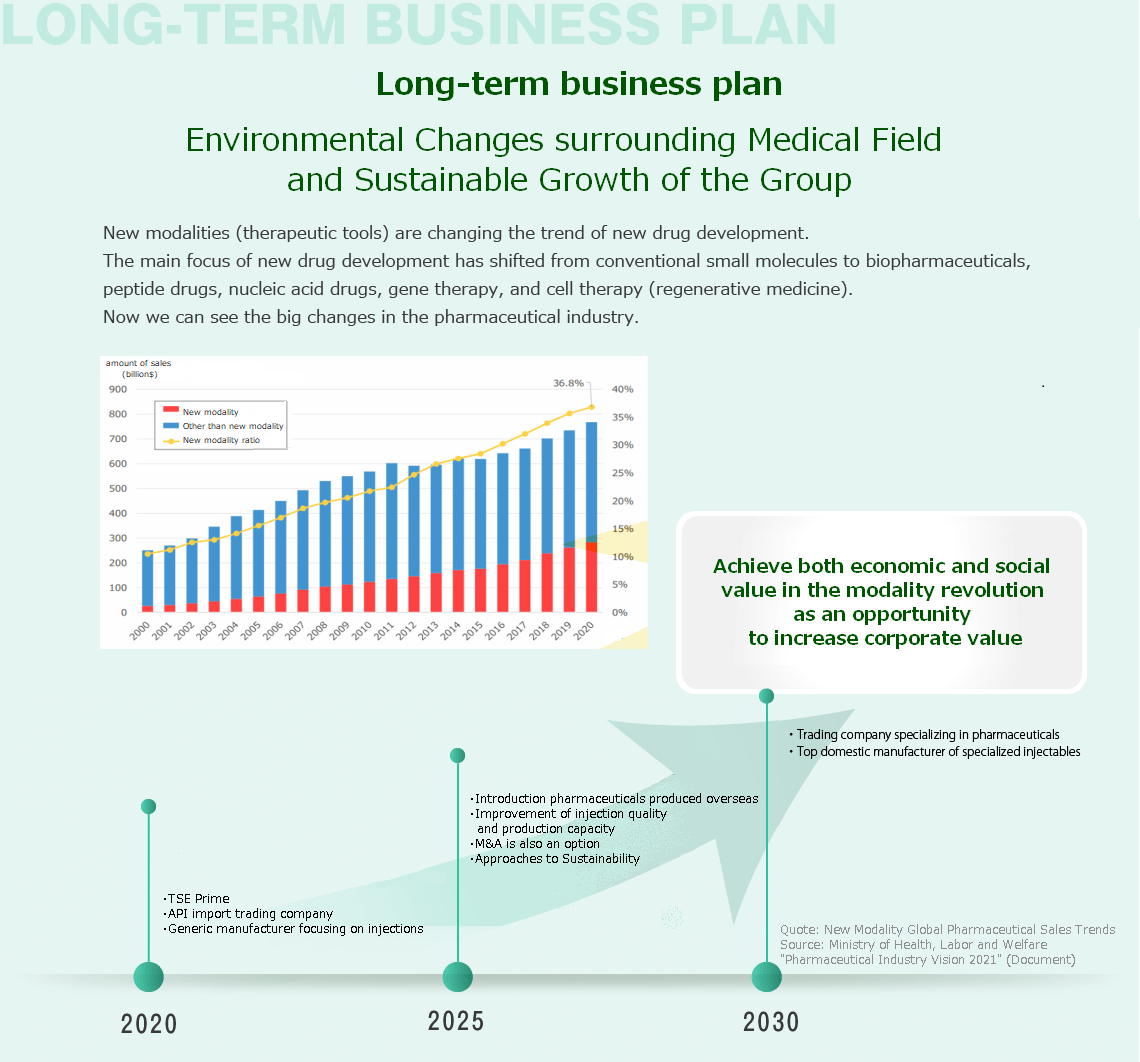
4Long-Term Business Plan
In the medical field, the focus on new drug development is shifting from conventional small molecules to polymers, such as biopharmaceuticals. Additionally, the modalities (treatment methods) are becoming increasingly diversified and complex, including medium-sized molecules, regenerative medicine, and gene therapy.
To enhance corporate value amidst such changes in the business environment, we need to continue implementing innovation to cope with these changes.
Consistent with the corporate slogan, "New Business Innovation," we will strive to improve our existing business model. However, we will further leverage the uniqueness of our business, which combines the functions of a trading company and a manufacturer, to provide a higher level of value to the domestic pharmaceutical industry.
In the API sales business, we aim to become a trading company specializing in APIs and importing pharmaceuticals produced overseas into Japan.
In the pharmaceutical manufacturing and sales business, we aim to become a top generic manufacturer of injectables by further improving quality and production capacity with M&A as an option.
As a company in the prime market, we will take the modality revolution as an opportunity to enhance corporate value by implementing sustainability initiatives to contribute to society, and we will continue to achieve sustainable growth by balancing economic and social value.
Business System Chart